Is ACANA approved by the fda

16 dog food brands may cause heart disease in pets, FDA warns
Fast facts:
- The U.S. Food and Drug Administration is still trying to determine why dogs eating certain types of pet food are seemingly more at risk of canine heart disease than others.
- Since alerting the public to the increasing caseload of dilated cardiomyopathy, or DCM, in dogs nearly a year ago, the FDA is for the first time identifying 16 pet food brands most frequently connected to the disease.
- Still, the agency said it has "not yet determined the nature of this potential link," and urged dog owners to consult with a veterinarian for advice on their pet's diet.
The U.S. Food and Drug Administration has identified more than a dozen brands of pet food it says are most frequently connected to a spike in reported cases of heart disease in dogs.
The FDA is continuing to investigate more than 500 reports of dilated cardiomyopathy, or DCM, in dogs eating certain types of pet food. A form of canine heart disease, DCM can cause congestive heart failure in dogs.
"We know it can be devastating to suddenly learn that your previously healthy pet has a potentially life-threatening disease like DCM," Steven Solomon, director of the FDA's Center for Veterinary Medicine, said Thursday in a statement.Because the FDA has "not yet determined the nature of this potential link, we continue to encourage consumers to work closely with their veterinarians."
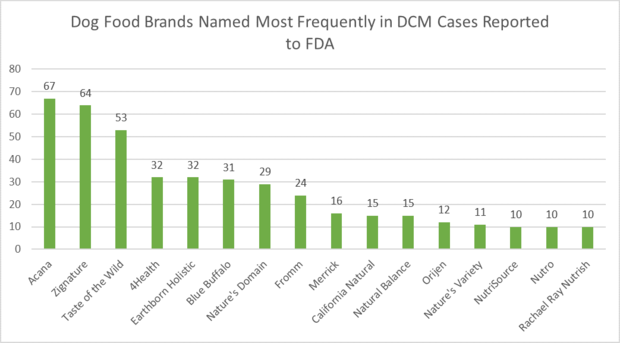
The FDA initially alerted the public to the cases plaguing dogs last July but did not specify food brands. The agency instead pointed to pet food labeled as "grain-free" and containing peas, lentils and other legume seeds and/or potatoes as their primary ingredients.
The probe now has the agency identifying 16 brands of dog food with the most frequently reported cases of DCM. Acana was named in 67 DCM reports, Zignature in 64 and Taste of the Wild in 53.
Pet food industry barks back
Zignature, for one, disputed any connection. "In parallel with the FDA investigation, our own third-party internal studies found no link between our high-quality pet food products and any of the other physical characteristics that correlate to DCM," Zignature said in apost on its site.
Champion Petfoods, which owns Arcana and Orijen, is working on its own and with others in the industry to try to determine the cause of DCM, but objected to the FDA's listing of brands.
"We think it is misleading for the FDA to post the names of brands, while at the same time fully stating that they have no scientific evidence linking diet to DCM. We feel this will only serve to further confuse pet lovers," the company stated.
The company's research shows "Champion pet foods are safe," it said.
The Pet Food Institute, a trade group that represents 98% of pet food and treat makers, said it has consulted with nutritionists, product safety experts and veterinarians for more than a year in trying to determine if there's a link between diet and DCM. "This is a complex issue with many factors requiring scientific evaluation," Dana Brooks, the group's president and CEO, said in a statement.
Noting that the FDA's probe focused on ingredients in grain-free pet food, the agency "has not identified any established link between certain ingredients and incidents of DCM," the industry group stated on its web site, which also noted "millions of dogs eat and are thriving on grain-free dog food."
The causes of DCM "may be the result of many factors, including a recipe formulation and processing, and your individual pet," according to the institute, which advised those with questions about their pet's food to contact the manufacturer and to consult with their family vet.
Between January 2014 and April 30, 2019, the FDA received 524 reports of DCM, including 119 dog deaths and five cat fatalities. Of those reports, 222 of them came between Dec. 1, 2018, and the end of April, the agency said.
Here is the list of 16 pet food brands and the number of reported DCM cases that the FDA suspects are related to each brand:
- Acana: 67
- Zignature: 64
- Taste of the Wild: 53
- 4Health: 32
- Earthborn Holistic: 32
- Blue Buffalo: 31
- Nature's Domain: 29
- Fromm: 24
- Merrick: 16
- California Natural: 15
- Natural Balance: 15
- Orijen: 12
- Nature's Variety: 11
- NutriSource: 10
- Nutro: 10
- Rachael Ray Nutrish: 10
Kate Gibson is a reporter for CBS MoneyWatch in New York.
ACANA receives its first FDA approval
AUBRUN, KY. ACANA pet food, owned by Champion Petfoods, announced May 18 that the ACANA Indoor Entre for cats has received approval by the US Food and Drug Administration (FDA) for hairball control. The FDAs approval confirms that the cat food helps to control hairballs within cats and is the first FDA claim for ACANA and Champion Petfoods.
The functional ACANA Indoor Entre is formulated with oat groats, Miscanthus grass, lentils, chickpeas and lentil fiber to provide an extra boost of fiber to help control hairballs. According to ACANA, because cats are self-groomers, they often ingest fur, which can accumulate and cause hairballs and vomiting that can prove harmful to their overall health and wellness.
"When a cat regurgitates a hairball, it can negatively impact their wellbeing and result in loss of nutrition, esophageal irritation, and other potential medical problems," said Darcia Kostiuk, DVM, in-house senior veterinarian at ACANA. "By increasing the fiber content, using oat groats, Miscanthus grass, lentils and chickpeas, ACANA Indoor Entre improves food digestibility and aids in the reduction of feline hairball symptoms."
ACANA Indoor Entre is developed specifically for indoor cats and is also formulated with chicken, turkey, herring and rabbit. The food is made without artificial colors, flavors or preservatives. The food originally launched at Global Pet Expo 2020, in which ACANA and Champion Petfoods showcased the formula along with a slew of other products.
"Our team at ACANA is dedicated to helping cats thrive and receiving FDA approval for hairball control demonstrates our commitment to going above and beyond when it comes to food safety," said Billy Frey, director of ACANA Cat at Champion Petfoods. "Pet lovers value the health and wellbeing of their feline friends, and ACANA Indoor Entre can support regular feline hairball control."
ACANA Indoor Entres packaging now includes the FDA approval statement of Helps control hairballs with fiber from oat groats, Miscanthus grass, lentils, chickpeas and lentil fiber. The cat food is available at independent pet retailers, as well as Petco, Chewy and Amazon.
Read morenews from pet food manufacturers.
U.S. Food and Drug Administration
December 23, 2022:
FDA does not intend to release further public updates until there is meaningful new scientific information to share. A count of reports of DCM in dogs submitted to FDA as of November 1, 2022, has been added to Questions & Answers: FDAs Work on Potential Causes of Non-Hereditary DCM in Dogs. FDA has followed up on a subset of these reports, but is unable to investigate every report to verify or confirm the reported information. While adverse event numbers can be a potential signal of an issue with an FDA regulated product, by themselves, they do not supply sufficient data to establish a causal relationship with reported product(s). FDA continues to encourage research and collaboration by academia, veterinarians, and industry.
Updated June 27, 2019
In July 2018, the FDA announced that it had begun investigating reports of canine dilated cardiomyopathy (DCM) in dogs eating certain pet foods, many labeled as "grain-free," which containeda high proportion of peas, lentils, other legume seeds (pulses), and/or potatoes in various forms (whole, flour, protein, etc.) as main ingredients (listed within the first 10 ingredients in the ingredient list, before vitamins and minerals). Many of these case reports included breeds of dogs not previously known to have a genetic predisposition to the disease. The FDAs Center for Veterinary Medicine (CVM) and the Veterinary Laboratory Investigation and Response Network (Vet-LIRN), a collaboration of government and veterinary diagnostic laboratories, continue to investigate this potential association. Based on the data collected and analyzed thus far, the agency believes that the potential association between diet and DCM in dogs is a complex scientific issue that may involve multiple factors.
We understand the concern that pet owners have about these reports: the illnesses can be severe, even fatal, and many cases report eating grain-free labeled pet food. The FDA is using a range of science-based investigative tools as it strives to learn more about this emergenceof DCM and its potential link to certain diets or ingredients.
Following an update in February 2019 that covered investigative activities through November 30, 2018, this is the FDAs third public report on the status of this investigation.
On this page:
Cases Reported to FDADiet Information from Reported CasesProduct TestingTaurine & Amino AcidsDiagnostic Testing Vet-LIRNCollaborationWhat you can doWhats NextAdditional Information
Cases Reported to FDA
For the purposes of this investigation, the FDA defines a case as an illness reported to FDA involving a dog or cat that includes a diagnosis of DCM. Many of the reports submitted to the FDA included extensive clinical information, including echocardiogram results, cardiology/veterinary records, and detailed diet histories. The numbers below only include reports in which the dog or cat was diagnosed with DCM by a veterinarian and/or veterinary cardiologist. We did not includein these numbersthe many general cardiac reports submitted to the FDA that did not have a DCM diagnosis. However, this case information is still valuable, as it may show heart changes that occur before a dog develops symptomatic DCM. (Please see the Vet-LIRN DCM Investigative Updatefor more technical information on the reported cases, including those without a formal diagnosis of DCM).Although the FDA first received a few sporadic reports of DCM as early as 2014, the vast majority of the reports were submitted after the agency notified the public about the potential DCM/diet issue in July 2018.
Between January 1, 2014 and April 30, 2019, the FDA received 524 reports of DCM (515canine reports, 9feline reports). Approximately 222of these were reported between December 1, 2018 and April 30, 2019 (219canine reports, 3 feline reports). Some of these reports involved more than one affected animal from the same household. The breakdown of reported illnesses below reflects the number of individual animals affected.The American Veterinary Medical Association estimates that there are 77 million pet dogs in the United States. Most dogs in the U.S. have been eating pet food without apparently developing DCM. Its not known how commonly dogs develop DCM, but the increase in reports to FDA signal a potential increase in cases of DCM in dogs not genetically predisposed.
Animal numbers in DCM Reports received between January 1, 2014 and April 30, 2019
| Number of animals affected | |||
|---|---|---|---|
| Dogs | 515 | 560 | 119 |
| Cats* | 9 | 14 | 5 |
*Cats are generally more likely to develop hypertrophic cardiomyopathy (a heart disease)
Dilated cardiomyopathy is recognized as a genetic condition in dogs, typically in large or giant breeds, such as the Doberman Pinscher, Great Dane, or the Irish Wolfhound. It is also seen in Cocker Spaniels associated with taurine deficiency. It is believed to be less common in small and medium breed dogs. We suspect that cases are underreported because animals are typically treated symptomatically, and diagnostic testing and treatment can be complex and costly to owners. FDA has observed a reporting bias for breeds like Golden Retrievers due to breed-specific social media groups and activities that have raised awareness of the issue in these communities and urged owners and vets to submit reports to FDA. Because the occurrence of different diseases in dogs and cats is not routinely tracked and there is no widespread surveillance system like the Centers for Disease Control and Prevention havefor human health, we do not have a measure of the typical rate of occurrence of disease apart from what is reported to the FDA.
Additional breeds with more than one report include Afghan Hound, Australian Cattle Dog, Beagle, Belgian Tervueren, Border Collie, Boston Terrier, Bull Terrier, Chihuahua, Dalmatian, English Cocker Spaniel, English Springer Spaniel, Flat-coated Retriever, French Bulldog, Gordon Setter, Hound (unspecified), Irish Setter, Irish Soft-Coated Wheaten Terrier, Jack Russel Terrier, Maltese, Miniature Schnauzer, Old English Sheepdog, Pomeranian, Portuguese Water Dog, Pug, Retriever (unspecified), Rhodesian Ridgeback, Rottweiler, Rough-haired Collie, Saluki, Samoyed, Schnauzer (unspecified), Shepherd (unspecified), Staffordshire Bull Terrier, Standard Long-haired Dachschund, Vizsla, Whippet, and Yorkshire Terrier.
Genetic forms of DCM tend to affect male large and giant breed dogs beginning in middle to older age. DCM cases reported to FDA CVM have involved a wide range of dog breeds, ages and weights. There have been a greater proportion of males than females, consistent with what is seen in genetic forms. The significance of this is unknown, but it may be that some cases are genetic in origin or a combination of diet and genetic tendencies.
Table 1: Mean Age and Weight - DCM Cases in Dogs Reported to FDA-CVM
| Age (years) | 6.6 | 0.4-16 |
| Weight (lbs) | 67.8 | 4-212 |
Table 2: Mean Age and Weight - DCM Cases in Cats Reported to FDA-CVM
| Age (years) | 6 | 0.4-17 |
| Weight (lbs) | 10.7 | 7-13 |
Table 3: Sex of DCM cases reported to FDA-CVM by species (%)
| Dogs | 58.7 | 41.3 |
| Cats | 62.5 | 37.5 |
Back to the top
Diet Information from Reported Cases
Review of the canine reports shows that most reports were for dry dog food formulations, but raw food, semi-moist food, and wet foods were also represented.
When examining the most commonly reported pet food brands named in DCM reports submitted to the FDA, it is important to note that the graph below is based on reports that included brand information and that some reports named multiple brands. Brands that were named ten or more times are featured below. For a granular, case-by-case breakdown of DCM reports submitted to the FDA, see Canine Dilated Cardiomyopathy Complaints Submitted to FDA-CVM Through April 30, 2019. FDA urges pet owners to work with their veterinarians, who may consult a board-certified veterinary nutritionist, to obtain the most appropriate dietary advice for their pet's specific needs prior to making diet changes.
To better characterize diets reported in DCM cases, product labels were examined to determine whether the product was grain-free (did not contain corn, soy, wheat, rice, barley or other grains), and whether the products contained peas, other lentils including chickpeas and beans, or potatoes (including sweet potatoes). Because so many products contained peas and/or lentils, a category was created for peas and/or lentils. More than 90 percent of products were grain-free, and 93 percent of reported products had peas and/or lentils. A far smaller proportion contained potatoes.
Animal protein sources in the reported diets varied widely, and many diets contained more than one protein source. The most common proteins in the reported diets were chicken lamb and fish; however, some diets contain atypical protein sources such as kangaroo, bison or duck.No one animal protein source was predominant.
Back to the top
Product Testing
Before the July 2018 DCM Update, FDA/Vet-LIRN had tested multiple products for minerals and metals (calcium, magnesium, phosphorus, iron, cobalt, copper, zinc, selenium, iodine) and amino acids including taurine, cysteine, and methionine. That product testing did not reveal any abnormalities.
Since the July 2018 DCM Update, Vet-LIRN tested both products labeled as "grain-free" and those containing grain for the following:
- protein, fat, moisture
- crude fiber, total dietary fiber, soluble fiber, insoluble fiber
- total starch, resistant starch
- cystine, methionine, and taurine
The average percent protein, fat, total taurine, total cystine, total methionine, total methionine-cystine, and resistant starch content on a dry matter basis (in other words, after removing all moisture content) were similar for both grain-free labeled and grain-containing products. For more details, please see the Vet-LIRN DCM Update.
Additional food testing is in progress.
Back to the top
Taurine & Amino Acids
Nutritional research indicates that taurine is generally not considered an essential amino acid for dogs,because these animalscan synthesize taurine from cysteine and methionine. Nearly all the grain-free products had methionine-cystine values above the minimum nutritional requirement of 0.65 percent for adult maintenance food for dogs published in the AAFCO Official Publication (OP).
The FDA is still gathering information to better understand if (and how) taurine metabolism (both absorption and excretion) may have a role in these reports of canine dilated cardiomyopathy.
Back to the top
Diagnostic Testing Vet-LIRN
Vet-LIRN has interviewed 95 owners of affected dogs and cats to document the pets complete dietary history and to explore any other factors that could have potentially contributed to development of DCM, such as environmental factors like heavy metal exposure or poisonous plant ingestion.
In addition, Vet-LIRN has contracted with a network lab to collect blood (whole blood and plasma), urine, feces, and DNA from dogs without a known breed predisposition to DCM (as a point of comparison) to send to Vet-LIRN for testing.
As of April 30, 2019, Vet-LIRN has reviewed results of 19gross necropsies from dogs with suspected heart disease, including 13necropsies that Vet-LIRN coordinated from cases reported through the FDA Safety Reporting Portal. There is one additional necropsy pending evaluation. The gross necropsies were performed by either veterinarians or veterinary pathologists, and Vet-LIRN is currently processing tissues for histopathology. A board-certified veterinary pathologist will review the histopathology slides.
Vet-LIRN has been collaborating with Chesapeake Veterinary Cardiology Associates (CVCA) to collect medical records, an owner interview, and diagnostic samples from pets with DCM that were diagnosed by a board-certified veterinary cardiologist by echocardiogram. These cases are included in the overall number of DCM cases, but were selected for further study because their ongoing program of care with the practice will be comprehensively documented and provided in full to Vet-LIRN.
Upon confirmation of a DCM diagnosis, CVCA collects blood (whole blood and plasma), urine, feces, DNA swabs, and food, if the pet is not receiving any supplements (e.g. taurine, cystine, or methionine) and is still eating a diet labeled grain-free. Vet-LIRN will test the blood and urine for taurine, cystine, methionine, and other amino acids. Vet-LIRN is archiving feces and DNA from these cases for possible future testing.
CVCA will repeat collection of urine, blood, and feces at 1 to 2 months, and at 6 months after the initial diagnosis and document any treatment or dietary changes, if any, that were recommended by the cardiologist. The repeat urine and blood samples will be tested for amino acid content and the feces archived. At the 6-month recheck, CVCA will also conduct a repeat echocardiogram to assess any changes to the heart. As of April 30, 2019, CVCA and Vet-LIRN have collected initial samples from 14 dogs, and 1 to 2-month samples from 10 dogs. CVCA is currently collecting the 6-month samples.
Of the original 14 dogs in this cohort, five dogs have been lost to follow-up at various points after the initial sample collection, including 4 deaths, and will not complete the sample collection. Vet-LIRN is currently evaluating the heart histopathology for two of the deceased dogs. The initial and 1 to 2-month blood and urine samples for 14 and 10 dogs, respectively, have been tested and are being evaluated.
Vet-LIRN is also collecting food associated with each CVCA case and will test each diet for:
- protein, fat, moisture
- crude fiber, total dietary fiber, soluble fiber, insoluble fiber
- total starch, resistant starch
- free and total cystine, methionine, and taurine
Golden Retrievers
Past publications and research suggest that Golden Retrievers may be genetically predisposed to taurine deficiency, which is well-documented as potentially leading to DCM.
Veterinary cardiologist Dr. Joshua Stern from the University of California at Davis has been studying the rise in cases of DCM in Golden Retrievers, including a potential dietary link. Many cases of DCM in Golden Retrievers are taurine-deficient. Pet owners who suspect their Golden Retrievers may be affected may wish to consult their veterinarian to discuss checking taurine levels or conducting an echocardiogram.
Back to the top
Collaboration
When unprecedented events such as these occur, the FDA often consults with stakeholders across the animal health community to help fill any knowledge gaps that may help inform its investigation. These collaborations can help provide pieces to complete the puzzle and allow us to gain a better understanding of what happened.
Veterinary Community
FDA veterinarians have been working with the veterinary community to exchange information about existing cases and the type of clinical information that is most helpful to the investigation. We are also consulting with a cadre of board-certified veterinary cardiologists and nutritionists to learn more about the presentation of these cases and how they respond to treatment.
Chesapeake Veterinary Cardiology Associates (CVCA), a multi-location veterinary cardiology practice based predominantly in the Mid-Atlantic states, has provided comprehensive records for some DCM cases (including medical records, owner interviews, and diagnostic samples from pets with DCM diagnosed with an echocardiogram by a board-certified cardiologist) to the Vet-LIRN network for further testing. These case records include imaging studies of the animals hearts, comprehensive dietary histories, diagnostic and treatment records, as well as outcomes of the cases.
FDA veterinarians have been working with Drs. Lisa Freeman of Tufts University, Joshua Stern of UC Davis and Darcy Adin of the University of Florida to learn more about their research findings and the cases theyve encountered. The three were contributing authors to a paper published in Journal of American Veterinary Medical Association in December 2018, Diet-associated dilated cardiomyopathy in dogs: what do we know?"
Pet Owners
As animal lovers and pet owners, FDA employees understand that the sudden onset of a life-threatening disease in a previously healthy pet can be devastating. The FDA is incredibly grateful to those pet owners who have agreed to be interviewed and given permission for their veterinarians to share medical records and diagnostic samples, including blood, serum and tissue. The agency is especially appreciative when pet owners make the difficult decision to provide tissues for analysis when a beloved pet passes away. The FDA believes that the information gained will help the FDA to understand the specific changes that are happening in the cardiovascular system and how they may relate to diet.
Industry
Another puzzling aspect of the recent spike in DCM cases is that they have occurred just in the last few years. The FDA is working with the pet food industry to better understand whether changes in ingredients, ingredient sourcing, processing or formulation may have contributed to the development of DCM.
Back to the top
What you can do
The FDA is open to additional opportunities for collaboration and welcomes the submission of any information that may aid in our investigation. Detailed instructions for submitting case information can be found on How to Report a Pet Food Complaint."
Pet Owners
If a dog is showing possible signs of DCM or other heart conditions, including decreased energy, cough, difficulty breathing and episodes of collapse, you should contact your veterinarian as soon as possible. If the symptoms are severe and your veterinarian is not available, you may need to seek emergency veterinary care. Your veterinarian may ask you for a thorough dietary history, including all the foods (including treats) the dog has eaten.
Veterinarians
CVM encourages veterinary professionals to report well-documented cases of DCM in dogs suspected of having a link to diet by using the electronic Safety Reporting Portal. The more information you are able to provide, particularly about feeding history, medical records, and diagnostic testing, the better. Detailed instructions can be found on How to Report a Pet Food Complaint." Technical veterinary information that may aid veterinarians can be found in our Vet-LIRN Update.
Industry
The FDA looks to industry organizations and pet food manufacturers to contribute to the FDA's investigation while continuingtheir own investigations to help shed light on potential issues with formulas or ingredients.
Back to the top
Whats Next
The FDA is continuing to investigate and gather more information in an effort to identify whether there is a specific dietary link to development of DCM and will provide updates to the public as information develops.
Additional Information
Back to the top









